Page 9 - hypertension_newsletter3
P. 9
REFLECTIONS rtensio n
Hypertension Global Newsletter #3
n oisnetr
Hype
hypertension. Both nocturnal hypertension and nondipping BP pattern have been associated with an increased risk of HMOHDype,
and adverse CV and kidney outcomes.
There has been some controversy and confusion regarding the optimal timing of dosing for antihypertensive medications, with
most clinical trials administering in the morning, but more recent studies suggesting that bedtime administration may be more
protective of CV outcomes. This position paper by the International Society of Hypertension (ISH) reviewed the published
evidence on the clinical relevance of diurnal variation in BP and the timing of antihypertensive drug treatment with the aim to
provide consensus recommendations for clinical practice.
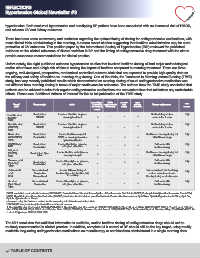
Unfortunately, the eight published outcome hypertension studies that involved bedtime dosing all had major methodological
and/or other flaws and a high risk of bias in testing the impact of bedtime compared to morning treatment. There are three
ongoing, well-designed, prospective, randomized controlled outcome trials that are expected to provide high-quality data on
the efficacy and safety of bedtime vs. morning drug dosing. One of the trials, the Treatment in Morning versus Evening (TIME)
study, has very recently published results which demonstrated an evening dosing of usual antihypertensive medication was
not different from morning dosing in terms of major cardiovascular outcomes. The authors from the TIME study concluded that
patients can be advised to take their regular antihypertensive medications at a convenient time that minimizes any undesirable
effects. Please see ‘Additional Articles of Interest’ for link to full publication of the TIME study.
Randomized Same Double- Hard Risk
blind primary of biasa
Study/ Study design Arms to bedtime drugs bedtime design outcome Study design issues
year High
Randomized 2 arms: =1 bedtime drugs vs. vs. morning & morning
Hermida, et al. PROBE morning treatment High
(HYGIA)1 dosing High
2019 Randomized
PROBE +- - Not fixed drug choices High
Hermida, et al. + same in the 2 arms High
(MAPEC)2 Randomized High
2010 Double-blind 2 arms: =1 bedtime drugs vs. + - - Not fixed drug choices High
morning treatment + same in the 2 arms
Black, et al. Randomized High
(CONVINCE)3 Double-blind 2 arms: Bedtime verapamil + - + Bedtime vs. morning dosing but
2003 COER vs. morning atenolol or - - + + with different drugs
Randomized Open + - -
HOPE Study4 label hydrochlorothiazide - - + + Both arms with
2000 - - - bedtime dosing
Randomized 2 arms: Ramipril vs. placebo, - - -
Tatti, et al. Double-blind both given at bedtime Bedtime vs. morning dosing but
(FACET)5 - with different drugs
1998 Not randomized 2 arms: Bedtime amlodipine vs.
Prospective morning fosinopril Both arms with
Staessen, et al. Observational + bedtime dosing
(Syst-Eur)6 2 arms: Nitrendipine vs. placebo,
1997 Not randomized both given at bedtime Not randomized. Not fixed drug
Prospective + choices same in each arm.
Sobiczewski, 4 arms: Different timing of
et al.7 treatment (morning, bedtime, bid, Morning dosing vs.all other arms
2014
tid or more) + Both arms with
Liu, et al. bedtime dosing
(Syst-China)8 2 arms: Nitrendipine vs. placebo,
1998 both given at bedtime
COER, controlled-onset extended-release; CONVINCE, Controlled Onset Verapamil Investigation of Cardiovascular End Points; FACET, Fosinopril Amlodipine Cardiovascular Events Trial;
HOPE, Heart Outcomes Prevention Evaluation; MAPEC, Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares; Syst-China, Systolic Hypertension in China; Syst-Eur,
Systolic Hypertension in Europe.
aAssessed using the Cochrane revised tool for assessing risk of bias in randomized trials.
bCONVINCE was prematurely terminated for commercial reasons.
1. Hermida RC, et al. Eur Heart J. 2020;41:4565-4576; 2. Hermida RC, et al. Chronobiol Int. 2010;27:1629-1651; 3. Black HR, et al. JAMA. 2003;289:2073-2082; 4. Yusuf S, et al. N Engl J
Med. 2000; 342:145-153; 5. Tatti P, et al. Diabetes Care. 1998;21:597-603; 6. Staessen JA, et al. Lancet. 1997;350:757-764; 7. Sobiczewski W, et al. Pharmacol Rep. 2014;66:448-452; 8. Liu
L, et al. J Hypertens. 1998;16:1823-1829.
The ISH concludes that until that information is available, routine bedtime dosing of antihypertensive drugs should not be
routinely recommended in clinical practice. In addition, complete 24 h control of BP should still be the key target, using readily
available long-acting antihypertensive medications as monotherapy or combinations administered in a single morning dose.
TABLE OF CONTENTS